|
Yasushi Shibuta, Ph.D. (ResearcherID:A-6301-2010) Associate Professor Department of Materials Engineering The University of Tokyo |
| Index Profile Research Publication & Presentation Etc Lab |
|
last update, 1 Apr, 2014
RESEARCHMy recent work has focused on understanding the nature of phase transition during synthesis and processing of materials by numerical modelling. Our target ranges from iron and steel to advanced materials such as carbon nanotube and metal nanoparticles. We are mainly using a classical molecular dynamics (MD) simulation. In addition, ab-initio calculation, phase-field method, moving particle simulation, and finite element method have been employed to meet the request of multi-scale modelling. TOPICS 1. Metal-catalyzed growth of carbon nanotubes and graphene 2. Understanding solidification and phase transformation from atomic point of view 3. Interaction of graphenes with a turbostratic orientational relationship 4. Phase-field modelling of electrochemical process Metal-Catalyzed Growth of Carbon Nanotubes and Graphene It is empirically known that the yield and quality of the carbon nanotubes and graphene products strongly depend on a choice of carbon source molecules and additive. Therefore, it is important to understand the dissociation process of carbon source molecules. We have investigated the dissociaton of methane on the nickel(111) surface to understand the initial stage of graphene growth via a CVD technique by ab initio molecular dynamics simulation [1]. 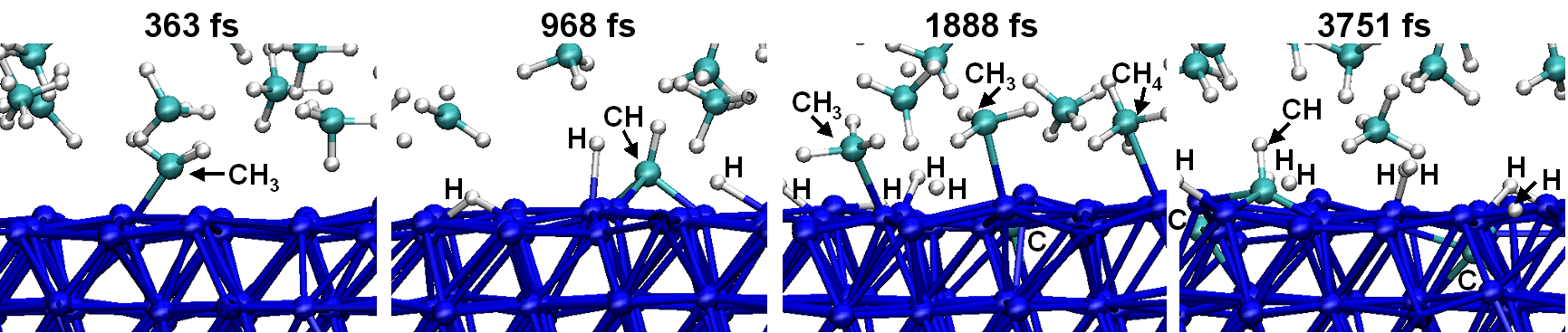 [1] Y. Shibuta, R. Alifin, K. Shimamura, T. Oguri, F. Shimojo, S. Yamaguchi, Chem. Phys. Lett. 565 (2013) 92-97. (collaborative work with Kumamoto University, Japan) Moreover, dissociation of ethanol on a nickel cluster is investigated to reveal the bond dissociation mechanism of carbon source molecules during carbon nanotube synthesis. It was found that C-C bonds in only CHxCO fragments are dissociated on the nickel cluster, whereas there is no preferential structure among the fragments for C-O bond dissociation. 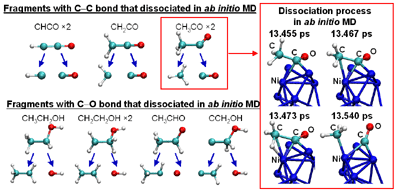 [2] T. Oguri, K. Shimamura, Y. Shibuta, F. Shimojo, S. Yamaguchi, Chem. Phys. Lett. 595-596 (2014) 185-191. (collaborative work with Kumamoto University, Japan) Regarding the subsequent cap formation process, Shibuta and Maruyama [3] reported a continuous simulation of formation process of an initial cap structure of a single-walled carbon nanotube (SWNT) by the classical MD method. In the simulation, randomly-distributed carbon atoms dissolved into nickel cluster until saturation, graphite networks precipitated and the cap was formed on the surface of the nickel cluster. 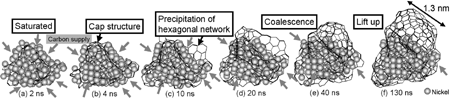 [3] Y. Shibuta, S. Maruyama, Chem. Phys. Lett. 382(2003)381-386. Followed by a number of simulation and combined with the support of in situ TEM observations, currently, these is a broad consensus on the metal-catalyzed cap growth model. One of the unsolved issues is to understand a key factor determining a chirality of the SWNT. Shibuta and Elliott [4] have investigated the role of catalytic metals on the SWNT growth by focusing on the orientation relationship between graphite network and crystalline metal surface.  [4] Y. Shibuta, J.A. Elliott, Chem. Phys. Lett. 472(2009)200-206. (collaborative work with University of Cambridge, UK) Understanding Solidification and Phase Transformation of Metals from Atomic Point of View The kinetics of a solid-liquid interface of iron with a triple point is investigated by a large-scale MD simulation performed on GPU [5]. A grain boundary groove evolves at the triple point of a solid-liquid interface with a bccâ░3(111) tilt grain boundary during solidification, whereas a solid-liquid interface with a twin boundary of small grain boundary energy is almost planar except in the vicinity of the triple point. 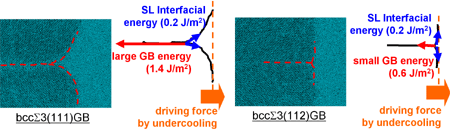 [5] Y. Shibuta, K. Oguchi, T. Suzuki, ISIJ Int. 52(2012)2205-2209. Phase transition in metal nanoparticles is an interesting issue since it is different from that in bulk metal. In addition to experiments over many years, molecular dynamics simulation contributes to interpret thermodynamic properties in the nanoparticles. Shibuta and Suzuki [6] have investigated the solidification and melting of metal nanoparticles and confirmed that the depression of the melting point of nanoparticles is proportional to the inverse of particle radius.  [6] Y. Shibuta, T. Suzuki, J. Chem. Phys. 129(2008)144102. Moreover, the kinetics of the (110)bcc//(111)fcc heterointerface of iron during the fcc-bcc phase transformation has been investigated [7]. The various orientation relationships (ORs) between Nishiyama-Wasserman (N-W) and Kurdjumow-Sachs (K-S) ORs, which are experimentally observed, were examined. The planar propagation of the heterointerface was observed in the case of the N-W and near N-W ORs, whereas a fast needlelike growth after planar growth was observed in the case of the K-S and near K-S ORs.  [7] S. Tateyama, Y. Shibuta, T. Suzuki, Scripta Materialia 59(2008)971-974. Interaction of Graphenes with a Turbostratic Orientational Relationship It is well known that the AB stacking order between graphene layers in graphite is energetically stable. However, there is little information on the interaction between the graphite layers with a turbostratic orientational relationship (OR). Shibuta and Elliott [8] have investigated the interaction of two rigid defect-free graphene sheets with various turbostratic ORs and revealed turbostratic orientations diminish the energy for translational displacement to zero. 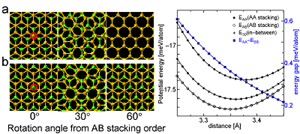 [8] Y. Shibuta, J.A. Elliott, Chem. Phys. Lett. 512(2011)146-150. (collaborative work with University of Cambridge, UK) Phase-Field Modelling of Electrochemical Process A phase-field (PF) model for electrochemical processes, in which cations were driven by an electrostatic potential coupled with a thermodynamic potential, was formulated on the basis of the PF model for binary alloys. Using this model, Cu electrodeposition from CuSO4 solution has been investigated [9]. 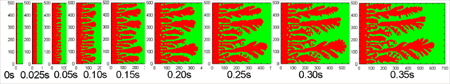 [9] Y. Shibuta, Y. Okajima, T. Suzuki, Sci. Tech. Adv. Mater. 8(2007)511-518. Above methodology is now employed for application of the electrorefining process of materials. Morphology of uranium electrodeposits on cathode with respect to applied voltage, zirconium concentration in the molten salt and the size of primary deposit during pyroprocessing is systematically investigated [10]. It is found that there is a threshold zirconium concentration in the molten salt demarcating planar and cellular/needle-like electrodeposits, which agrees with experimental results. 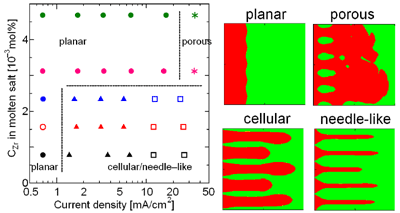 [10] Y. Shibuta, T. Sato, T. Suzuki, H. Ohta, M. Kurata, J. Nuclear Materials. 436(2013) 61-67. (collaborative work with JAEA and CRIEPI, Japan) |